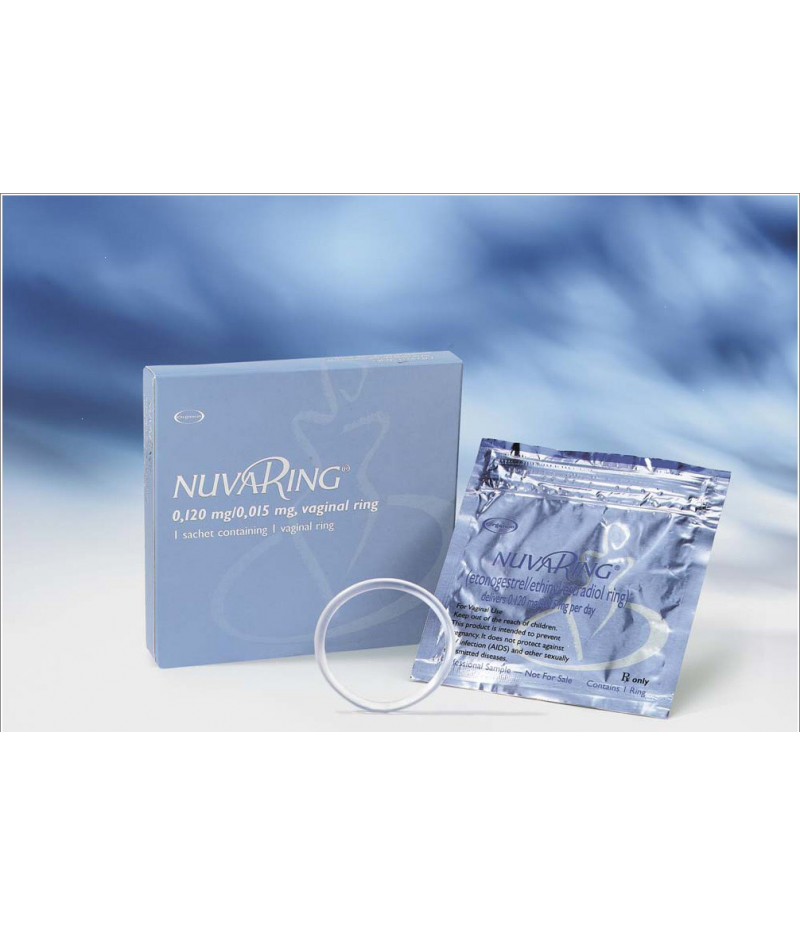
NovaRing
- $59.67
- 2 or more $58.50
- 3 or more $57.64
- Availability:In Stock
NovaRing instruction for useReed more about NovaRing and buy it onlineComposition1 ring contains ethinylestradiol 2.7 mg and etonogestrel 11.7 mg; in the form of auxiliary substances: ethylene and vin
NovaRing instruction for use
Reed more about NovaRing and buy it online
Composition
1 ring contains ethinylestradiol 2.7 mg and etonogestrel 11.7 mg; in the form of auxiliary substances: ethylene and vinyl acetate copolymer, magnesium stearate.
Form of issue
The vaginal ring is colorless, transparent, smooth, at the junction - a transparent area. In a waterproof aluminum foil bag.
pharmachologic effect
Hormonal contraceptive drug.
Etonogestrel is a progestogen that binds to target organs with progesterone receptors.
Ethinyl estradiol is an estrogen. The effect of the drug is provided by a combination of various mechanisms, the most significant of which is suppression of ovulation.
The use of the drug regulates the menstrual cycle, reducing pain in menstrual bleeding and their intensity. This reduces the likelihood of developing iron deficiency anemia.
The use of the drug reduces the risk of ovarian and endometrial cancer, development of ovarian cysts, ectopic pregnancy, inflammation of the female reproductive system and benign breast pathologies.
Pharmacodynamics and pharmacokinetics
Etonogestrel is absorbed through the vaginal mucosa and its maximum concentration is reached after about 7 days. Bioavailability is about 100%, which is higher than with oral etonogestrel. The substance is metabolized in the liver and excreted in the urine and bile. The half-life of metabolites is 6 days.
Ethinyl estradiol also penetrates the vaginal mucosa into the bloodstream. The maximum concentration is achieved after 3 days. Bioavailability is approximately 56% and is comparable to oral intake. Metabolized with urine and bile, the half-life is about 36 hours.
Indications for use
Contraception.
Contraindications
arterial and venous thrombosis, thromboembolism and predisposition to them;
heart defects with complications in the form of thromboses;
migraine;
diabetes mellitus with vascular changes;
pancreatitis;
severe liver disease;
malignant and benign liver tumors;
hormone-dependent malignant tumors (mammary gland and reproductive system);
vaginal bleeding of unknown origin;
pregnancy;
hypersensitivity to any of the substances in the drug (acting or ancillary).
If any of these conditions occur, stop taking the drug immediately.
Prescribe the drug with caution in such cases:
presence in the family history of venous or arterial thrombosis;
large surgical interventions and interventions on the lower limbs, prolonged immobilization;
obesity with a body mass index of more than 30;
thrombophlebitis;
arterial hypertension;
Smoking (especially for women over 35);
impaired lipid metabolism;
heart defects;
atrial flutter;
diabetes;
violations of the liver;
cholelithiasis;
hemolytic-uremic syndrome;
chorea;
otosclerosis with loss of hearing;
systemic lupus erythematosus;
angioedema;
ulcerative colitis and Crohn's disease;
sickle-cell anemia;
porphyria;
Chloasma;
cases of difficulty in using the vaginal ring: severe chronic constipation, a bladder and rectum hernia, cervical prolapse.
In case of worsening of the disease and worsening of the condition, or when the condition first arises from the above, the further question of taking the drug must be solved with the doctor.
Side effects
When used, the following NovaRing side effects may occur, occurring at different frequencies.
Infections: vaginal infection and urinary tract, cervicitis.
Immune system: hypersensitivity.
Metabolism: increased appetite and weight gain.
Mental disorders: decreased sexual desire, mood changes, depression.
Nervous system: headache, dizziness, migraine.
Organs of vision: visual impairment.
Cardiovascular system: increased blood pressure, thromboembolism, sensation of "hot flashes".
Digestive system: nausea, bloating, abdominal pain, diarrhea, constipation, vomiting.
Skin: acne, itching, rash.
Musculoskeletal system: pain in the limbs and back area, spasms in the muscles.
Urinary system: dysuria, frequent urination.
On the part of the reproductive system and mammary glands, the side effects of the ring can be manifested by: engorgement and soreness of the mammary glands, genital itching, dysmenorrhea, vaginal discharge, amenorrhea, spotting during intercourse, heavy menstruation, uterine bleeding, premenstrual syndrome, burning in the vagina, painful sensations and discomfort in the vagina.
General condition of the body: fatigue, swelling.
May also be noted discomfort and discomfort in the vagina.
Instructions for use Novaring (Method and dosage)
The ring is injected once every 4 weeks into the vagina, where it is 21 days, and then removed. A new ring is injected after a break of 7 days.
On the 2nd-3rd day after removal of the ring, bleeding starts, which is associated with the cessation of the drug.
If hormonal contraceptives were not used in the previous menstrual cycle, NovaRing contraceptive ring is administered on the first day of menstruation. It is possible to install up to 5 days of the cycle, but in this case the first week of using the drug is recommended to use a condom.
When switching from combined oral contraceptives, the ring is administered on the last day of the interval between taking hormonal contraceptives. With the correct and regular intake of a combined hormonal contraceptive and confidence in the absence of pregnancy, you can insert the vaginal ring on any day of the cycle.
When switching from a mini-saw, the ring can be used any day (on the day of removal of the IUD or on the day of the next injection). In such cases it is necessary to use barrier contraceptives within 1 week.
The instruction for Novaring allows the use of the ring immediately after the abortion, carried out in the first trimester of pregnancy.
After abortion in the second trimester or delivery, use is allowed to begin on the 4th week after the abortion or childbirth.
The contraceptive effect can be disrupted if the regime and rules of application are not observed.
In the event that there were sexual intercourse during the break in the use of the ring, pregnancy should be excluded.
When the ring ruptures, the release of hormones does not change. In this case, the ring, as a rule, falls out of the vagina. It must be replaced with a new one.
Rules for the use of NovaRing
The ring can be inserted into the vagina. For the introduction, it is necessary to choose a comfortable position - standing, with one leg raised, squatting or lying down. In this case, the ring should be squeezed and inserted into the vagina.
To remove the ring, it is compressed with the index and middle fingers and is taken out of the vagina.
Overdose
Serious consequences of an overdose were not observed.
There may be minor vaginal bleeding in girls, nausea or vomiting. For treatment, symptomatic therapy is performed.
Interaction
There may be interaction, manifested in the onset of acyclic bleeding, with the following drugs: barbiturates, carbamazepine, phenytoin, rifampicin, oxcarbazepine, topiramate, felbamate, preparations of St. John's wort perfumed. During treatment with these drugs and for 1 month after stopping therapy, they need to use a condom.
When treating antibiotics with ampicillins and tetracyclines, you need to use a condom during treatment and for 1 week after the antibiotic cancellation, because the effectiveness of contraceptives that contain ethinyl estradiol may decrease.
The use of suppositories with antifungal agents slightly increases the risk of damage and rupture of the ring.
The use of hormonal contraceptives can cause a change in the metabolism of other drugs, and their concentration in plasma or peripheral tissues may increase (cyclosporine) or decrease (lamotrigine).
Storage conditions
Keep out of the reach of children and at a temperature of at least 2 ° C and not higher than 8 ° C.
Shelf life - 3 years.
special instructions
The intake of hormonal contraceptives can be associated with the development of venous and arterial thrombosis.
The likely symptoms of thrombosis are:
pain and swelling in one leg;
sudden intense pain in the chest;
shortness of breath or cough of a paroxysmal nature;
dizziness, intense headaches;
sudden loss of vision or double vision;
speech impairment;
sudden weakness or numbness of one side of the body or some part of the body;
disturbance of movement.
If there are signs of thrombosis, stop taking hormonal contraceptives immediately and consult a doctor.
The reason for stopping hormonal contraceptives should be an increase in the severity of migraine and the frequency of seizures.
In women with hypertriglyceridemia, the risk of developing pancreatitis increases.
With a predisposition to the development of chloasma, it is necessary to avoid exposure to ultraviolet radiation and sunlight during the application of the ring.
The use of the drug may cause acyclic bleeding in the form of smearing discharge or bleeding that occurs suddenly.
Analogues
There are analogues for the pharmacological group, which include combined oral contraceptives: Midiana, Yarina.
Children
The safety of the NovaRing drug in adolescents under 18 years old has not been studied.
In pregnancy and lactation
NovaRing is intended to prevent pregnancy.
Use during pregnancy is contraindicated. When it comes, remove the ring.
Pregnancy after the cancellation of Novaring occurs when the natural cycle is restored and normal ovulation occurs.
The use of the drug during breastfeeding is contraindicated.
Reviews of the NovaRing
Many women turn to a medical forum with questions about applying the NovaRing. The doctors' comments on NovaRinge are mostly a reliable and effective remedy with a minimum of side effects. In none of the reviews was there mention of the onset of an unwanted pregnancy when taking the drug. The hormonal ring by gynecologists is widely used in endometriosis as a factor restraining the development of the disease. Some women report side effects in the form of discomfort in the vagina, weight gain and headache.