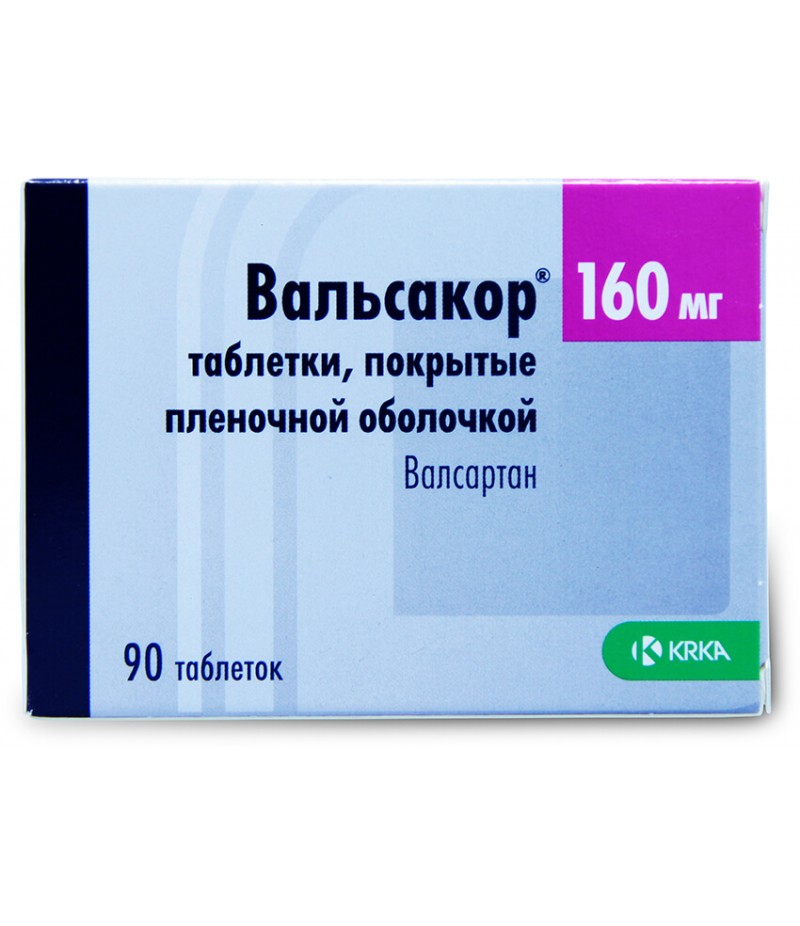
Edarbi KLO tabs 40mg + 12.5mg #28
- $38.46
- 3 or more $37.99
- Availability:In Stock
Edarbi KLO instruction for useReed more and buy Edarbi KLO hereCompositionFilm Coated Tabletsazilsartan medoxomil potassium 42.68 mg (equivalent to 40 mg azilsartan medoxomil)chlorthalidone 12.5 mgexcipients: mannitol - 211.23 mg;..
Tags: tabs
Edarbi KLO instruction for use
Reed more and buy Edarbi KLO here
Composition
Film Coated Tablets
azilsartan medoxomil potassium 42.68 mg (equivalent to 40 mg azilsartan medoxomil)
chlorthalidone 12.5 mg
excipients: mannitol - 211.23 mg; MCC - 54 mg; fumaric acid - 2 mg; sodium hydroxide - 0,69 mg; hyprolosis - 10.8 mg; Crospovidone - 22.5 mg; magnesium stearate - 3.6 mg
film cover: hypromellose 2910 - 7.8 mg; talc - 1.2 mg; titanium dioxide - 0.99 mg; iron dye red oxide - 0.01 mg; macrogol 8000 - 0.18 mg; gray ink F1 cleaned for labeling (shellac - 26%, iron dye black oxide - 10%, butyl alcohol - 38%, ethanol - 26%) - trace amounts.
pharmachologic effect
Pharmacological action - diuretic, antihypertensive, blocking AT1-receptors.
Dosage and administration
Inside, 1 time per day, regardless of meal times.
The recommended initial dose of Edarbi KLO is 40 mg of azilsartan medoxomil + 12.5 mg of chlorthalidone 1 time per day. If it is necessary to further reduce the blood pressure, the dose of Edarbi KLO can be increased to a maximum of 40 mg of azilsartan medoxomil + 25 mg of chlorthalidone 1 time per day.
Duration of treatment. Edarbi KLO should be taken daily, without interruption. In case of discontinuation of treatment, the patient should inform the doctor about it.
Special patient groups
Elderly patients (65 years and older). Correction of the initial dose of Edarbi KLO in elderly patients is not required.
Patients with impaired renal function. There is no clinical experience with the use of Edarbi KLO in patients with arterial hypertension (AH) with severe renal dysfunction (Cl creatinine less than 30 ml / min), so it is not recommended to use the drug in this category of patients.
Correction of the dosing regimen is not required in patients with impaired mild to moderate renal function (Cl creatinine more than 30 ml / min).
Patients with impaired liver function. The use of the drug in patients with severe hepatic impairment is not recommended due to the lack of clinical experience. Due to limited experience, Edarbi KLO should be used with caution in patients with mild or moderately impaired liver function (less than 9 on the Child-Pugh scale), since even small violations of water-electrolyte balance when taking diuretics can provoke hepatic coma. It is recommended to actively monitor the condition of such patients.
Reduced bcc. It is necessary to compensate for the loss of fluid and electrolytes in patients with reduced BCC before starting to use Edarbi KLO.
Heart failure. Due to the lack of clinical experience, Edarbi Klo should be used with caution in patients with hypertension with severe CHF (functional class IVHA classification NYHA).
Negroid race. Dose adjustment is not required, since The antihypertensive effect of Edarbi KLO in patients of the Negroid race is similar to its effect in patients of other races.
Skip dose
In case of skipping the next dose, the next dose should be taken at the usual time. You should not take a double dose of Edarbi KLO. Withdrawal syndrome (a sharp increase in blood pressure after discontinuation of the drug) due to the sudden cancellation of long-term therapy (within 6 months) of azilsartan medoxomil was not observed.
Release form
Tablets, film coated, 40 mg + 12.5 mg, and 40 mg + 25 mg.
In the case when the packaging is carried out by AndersonBrecon Inc., USA, which produces quality control - Takeda Pharmaceutical Company Limited, Japan: 7 tabl. in an aluminum blister with a desiccant incorporated in the PE layer. 4 bl. placed in a cardboard box.
In the case that packaging and quality control are carried out by Takeda Island Limited, Ireland: 14 tab. in an aluminum blister with a desiccant incorporated in the PE layer. 2 bl. placed in a cardboard box.
Storage conditions of Edarbi KLO drug
At temperatures not higher than 25 ° C, in the original packaging.
Keep out of the reach of children.
Shelf life of the drug Edarbi KLO - 3 years.
Terms of sell
You can buy Edarbi KLO without a prescription.