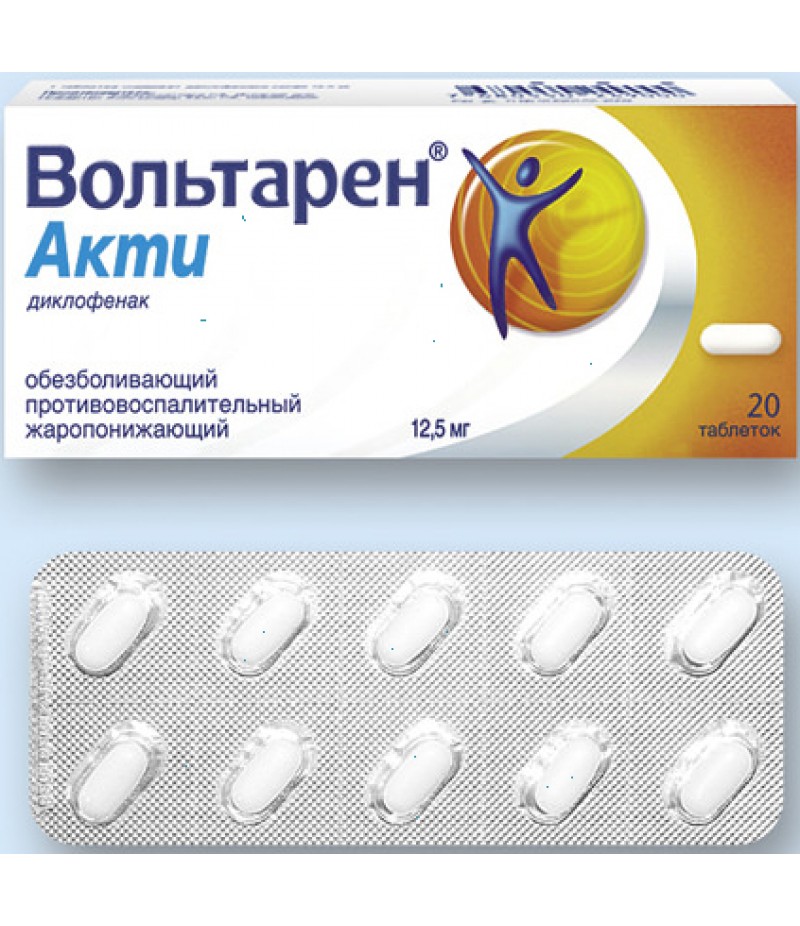
Voltaren acti tabs 12.5mg #20
- $12.15
- 3 or more $11.90
- Availability:Out Of Stock
Voltaren acti tabs instruction for useYou can buy Voltaren acti on this pageClinical and pharmacological groupNSAIDsForm of release, composition and packagingTablets covered with a film coating of whi
Tags: tabs
Voltaren acti tabs instruction for use
You can buy Voltaren acti on this page
Clinical and pharmacological group
NSAIDs
Form of release, composition and packaging
Tablets covered with a film coating of white color, oblong, with a smooth surface.
1 tab.
diclofenac potassium 12.5 mg,
which corresponds to the content of diclofenac 11.08 mg
Auxiliary substances: silicon dioxide - 8.025 mg, lactose monohydrate - 33.45 mg, corn starch - 99.75-101.745 mg, sodium carboxymethyl starch - 26.7 mg, povidone K30 - 4.05 mg, microcrystalline cellulose - 13.5 mg, magnesium stearate - 2.025 mg.
Composition of the coating: white coating mixture of septiphilm LP 770 - 6 mg (hypromellose - 3.9 mg, microcrystalline cellulose - 600 μg, stearic acid - 600 μg, titanium dioxide (E171) - 900 μg).
10 pieces. - blisters (1) - packs of cardboard.
10 pieces. - blisters (2) - packs of cardboard.
pharmachologic effect
NSAIDs. Has a pronounced analgesic, anti-inflammatory and antipyretic effect.
The mechanism of action is based on inhibition of the activity of cyclooxygenases (COX-1 and COX-2) followed by suppression of the inflammatory process, reduction of pain and fever.
After taking the Voltaren Acti tablets, the therapeutic effect develops after 15 minutes, which makes the use of the drug effective for rapid relief of pain and decrease in fever.
Pharmacokinetics
Data on the pharmacokinetics of Voltaren Acti are not available.
Indications
Removal and reduction of pain sensations of different origin, including:
- pain in muscles and joints (including back pain in different parts of the spine);
- headache and toothache;
- Pain during menstruation.
To eliminate the symptoms of colds and flu:
- pain in the muscles, joints;
- Sore throat;
- fever.
Contraindications
- attacks of bronchial asthma, urticaria or acute rhinitis in history caused by the use of acetylsalicylic acid or other NSAIDs (for example, ibuprofen);
- period after aortocoronary shunting;
- Stomach ulcer or ulcerative bowel disease in the phase of exacerbation;
- Ulcer bleeding or perforation;
- severe renal failure;
Severe hepatic impairment;
- severe heart failure;
- III trimester of pregnancy;
- children's age till 14 years;
- Hypersensitivity to the components of the drug.
With caution should be used in patients with IHD, cerebrovascular disease, congestive heart failure, dyslipidemia / hyperlipidemia, diabetes mellitus, peripheral arterial disease, with CC <60 ml / min, history of the development of gastrointestinal lesions, with prolonged use of NSAIDs , in patients with severe somatic diseases, with concomitant therapy with selective serotonin reuptake inhibitors, in elderly patients, smokers, who frequently use alcohol ь.
Dosage
The drug is prescribed inside.
For adults and adolescents older than 14 years, the initial dose is 25 mg (2 tablets) followed by 12.5-25 mg (1-2 tablets) every 4-6 hours as needed. The maximum daily dose is 75 mg (6 tablets).
Tablets should be taken whole, not liquid and squeezed with water. To achieve maximum therapeutic effect, the drug should be taken before meals.
Without a doctor's appointment, the duration of the Voltaren Akti application for fever is 3 days, with the pain syndrome - 5 days.
Side effects
Determination of the frequency of side effects: often (> 1/100, <1/10), sometimes (> 1/1000, <1/100), rarely (> 1/10 000, <1/1000), very rarely (<1 / 10 000, including separate messages).
On the part of the digestive system: often - nausea, vomiting, diarrhea, dyspepsia, abdominal pain, flatulence, anorexia, increased levels of hepatic transaminases; rarely - gastritis, gastrointestinal bleeding, bloody vomiting, diarrhea, melena with blood, stomach / intestinal ulcers (with / without bleeding or perforation), hepatitis, jaundice; very rarely - colitis (including colitis with blood, exacerbation of ulcerative colitis or Crohn's disease), constipation, stomatitis, glossitis, esophageal pathology, stricture of the esophageal opening of the diaphragm, pancreatitis, fulminant hepatitis.
From the nervous system: often - headache, dizziness; rarely - drowsiness; very rarely - paresthesia, memory impairment, convulsions, anxiety, tremor, aseptic meningitis, taste disorders, cerebrovascular disorders, loss of orientation, depression, insomnia, nightmares, irritability, psychotic disorders.
From the skin and subcutaneous tissues: often - a rash; very rarely - rashes in the form of blisters, eczema, erythroderma (exfoliative dermatitis), hair loss, photosensitivity.
From the hemopoietic system: very rarely - thrombocytopenia, leukopenia, anemia (including hemolytic and aplastic anemia), agranulocytosis.
Allergic reactions: rarely - hives, anaphylactic and anaphylactoid reactions (including arterial hypotension and shock); very rarely erythema multiforme, Stevens-Johnson syndrome, Lyell syndrome (toxic epidermal necrolysis), purpura, including allergic purpura, angioedema (including facial edema).
From the senses: very rarely - visual disturbances (impaired focus, diplopia), ringing in the ears, hearing impairment.
From the cardiovascular system: very rarely - a feeling of palpitations, sore throat, heart failure, myocardial infarction, arterial hypertension, vasculitis.
From the respiratory system: rarely - bronchial asthma (including dyspnea); very rarely - pneumonia.
From the side of the urinary system: very rarely - acute renal failure, hematuria and proteinuria, interstitial nephritis; nephrotic syndrome, papillary necrosis.
Other: rarely - swelling.
Overdose
Symptoms: increased blood pressure, renal failure, convulsions, respiratory depression, gastrointestinal complications.
Treatment: gastric lavage, reception of activated charcoal; the conduct of symptomatic therapy and measures aimed at maintaining the functions of the body.
The use of diuretics, infusion solutions, dialysis is ineffective when it is necessary to remove NSAID from the body, since the drugs of this group are characterized by a high degree of binding to plasma proteins.
Drug Interactions
With the simultaneous use of lithium and digoxin drugs, an increase in the concentration of lithium and digoxin in plasma is possible.
Diclofenac (as well as other NSAIDs) with simultaneous administration with diuretic or antihypertensive agents (eg, beta-blockers, ACE inhibitors) can reduce the severity of antihypertensive action. Simultaneous use of potassium-sparing diuretics can lead to an increase in serum potassium levels.
Simultaneous use of other systemic NSAIDs or corticosteroids leads to an increase in the number of side effects.
With the simultaneous administration of diclofenac with anticoagulants and platelet aggregation inhibitors, the risk of bleeding increases (using this combination requires extreme caution).
In clinical studies, it has been established that simultaneous use of diclofenac and oral hypoglycemic agents is possible, but the efficacy of the latter does not change. However, individual cases of development, both hypoglycemia and hyperglycemia, in which a dose adjustment of hypoglycemic drugs was required, are described.
Caution should be exercised with Voltaren Acti at intervals of less than 24 h before or after taking methotrexate, as the concentration of methotrexate in the blood may increase, which will lead to an increase in its toxicity.
With the simultaneous use of cyclosporine, the effect of NSAIDs on renal prostaglandins can lead to an increase in the nephrotoxicity of cyclosporine.
There are separate reports on the development of seizures caused by the combined use of quinolones and NSAIDs.
special instructions
When taking NSAIDs, there is a possibility of bleeding from the gastrointestinal tract, ulcerative lesions of the gastrointestinal tract, sometimes complicated by perforation, without previous warning signs or the presence of such attacks in the patient's anamnesis. These complications can have serious consequences especially for the elderly. If these symptoms occur, the drug should be immediately discontinued.
The risk of developing bleeding from the gastrointestinal tract increases with increasing doses of NSAIDs in patients with a history of peptic ulcer, especially if the disease is complicated by bleeding and perforation, as well as in elderly patients. To reduce the risk of complications, therapy should be initiated and maintained at the lowest effective dose level, taking into account the possibility of using combination therapy using agents that have protective effects on the gastric mucosa (eg, proton pump inhibitors or misoprostol).
When prescribing diclofenac, patients with an existing gastrointestinal pathology (ulcerative lesions, bleeding, perforation) in the history should be treated with careful medical supervision and observance of special care.
Caution is recommended for patients who simultaneously take medications that may increase the risk of ulcers or bleeding of the gastrointestinal tract, such as systemic corticosteroids, anticoagulants, platelet aggregation inhibitors, or selective serotonin reuptake inhibitors.
Patients suffering from ulcerative colitis or Crohn's disease should be treated under close medical supervision. as a result of diclofenac, there may be an exacerbation of these diseases.
The use of diclofenac should be discontinued at the first signs of skin rash, lesions of the mucous membranes and when other signs of hypersensitivity occur.
In the application of diclofenac, as well as other NSAIDs, in rare cases, allergies may occur, incl. anaphylactic / anaphylactoid reactions in patients who did not previously use diclofenac.
Diclofenac (as well as other NSAIDs) in connection with its pharmacological properties can mask the symptoms characteristic of infectious diseases.
The simultaneous use of diclofenac with systemic NSAIDs (including selective COX-2 inhibitors) should be avoided, since there is no evidence of a beneficial effect as a result of synergy, and there are no data on possible side effects.
Care should be taken when prescribing the drug to elderly patients. Weak or low-fat elderly patients are recommended to prescribe the drug at the lowest effective dose.
In the tablets Voltaren Akti contains lactose, therefore the drug is not recommended for patients with rare congenital diseases associated with impaired tolerance to galactose, severe lactase deficiency, and impaired absorption of glucose-galactose.
Patients suffering from bronchial asthma, seasonal allergic rhinitis, nasal mucosa edema (polygous nasal mucosa), chronic obstructive pulmonary disease or chronic respiratory tract infection (especially associated with allergic rhinitis-like symptoms), reactions to drugs from the NSAID group in the form of asthma attacks so-called "aspirin" asthma), Quincke's edema or urticaria develop more often than usual. Such patients are advised to take special care (readiness for urgent medical measures).
When diclofenac is prescribed, patients with impaired liver function should be closely monitored, as the condition of such patients may worsen. Against the background of the use of the drug Voltaren Acti, as well as other NSAIDs, the level of one or several hepatic enzymes may increase. Therefore, long-term therapy shows a regular study of liver function. If violations of the liver's functional parameters persist or worsen, or if complaints or symptoms that indicate liver disease develop, and if other adverse reactions (including eosinophilia, rash) occur, the drug should be discontinued. It should be borne in mind that hepatitis against diclofenac may occur without prodromal phenomena.
Care should be taken in patients with hepatic porphyria, as taking diclofenac can provoke an attack.
Since NSAIDs have been reported on fluid retention and the appearance of edema, special care should be taken in patients with impaired renal and cardiac function, history of arterial hypertension, elderly patients, concurrent administration of diuretics or drugs that have a significant effect on kidney function, and also to patients with a significant decrease in BCC of any etiology, for example, in the period before and after massive surgical interventions. In such cases, when diclofenac is used as a precautionary measure, monitoring of renal function is recommended. After discontinuing therapy, the baseline parameters are usually restored.
Voltaren Acti is recommended to be used for a short time, for several days. When the drug is prescribed for a longer time, a systematic control of the peripheral blood pattern is shown.
Voltaren Acti, like other NSAIDs, may temporarily inhibit platelet aggregation. Therefore, patients with hemostasis disorders need careful monitoring of relevant laboratory parameters.
Diclofenac, like other NSAIDs, can have a negative effect on female fertility, so it is not recommended to use the drug for women planning a pregnancy.
When taking Voltaren Akti while eating, the absorption of diclofenac decreases. Therefore, the drug is not recommended to be taken during or immediately after a meal.
Impact on the ability to drive vehicles and manage mechanisms
During the period of treatment, a slight decrease in the rate of psychomotor reactions is possible. Patients experiencing dizziness or other unwanted reactions from the side of the CNS, including visual impairment, should not drive vehicles or operate machinery.
Pregnancy and lactemia
Adequate and strictly controlled studies on the effects of diclofenac in pregnancy have not been conducted. Therefore, in the I and II trimesters of pregnancy, the drug is prescribed only in cases where the potential benefit of therapy for the mother exceeds the increased risk for the fetus.
The use of diclofenac, like other NSAIDs, is contraindicated in the third trimester of pregnancy because of possible atony of the uterus and / or premature closure of the ductus arteriosus.
Diclofenac, like other NSAIDs, is excreted in breast milk in small amounts. Therefore Voltaren Acti is not recommended for use during breastfeeding to prevent unwanted effects in the child.
Application in childhood
Contraindicated: children under 14 years.
In case of violations of kidney function
Given the important role of prostaglandins in maintaining renal blood circulation, caution should be exercised in patients with renal insufficiency. In such cases, it is necessary to monitor kidney function. The discontinuation of Voltaren Acti usually leads to the restoration of impaired renal function.
With violations of liver function
Patients with violations of the liver function or instructions to them in a history require strict medical supervision. When using the drug, it is possible to increase the serum levels of one or more liver enzymes. If prolonged use of diclofenac is necessary as a prophylaxis, liver function should be monitored regularly. If the liver is damaged or if there are other lesions (eosinophilia, rash), the drug should be stopped. The development of hepatitis is possible without prodromal symptoms.
Application in old age
With caution should be used in elderly patients.
Conditions of leave from pharmacies
The drug is dispensed without a prescription.
Terms and conditions of storage
The drug should be stored out of reach of children at a temperature of no higher than 25 ° C. Shelf life - 3 years.