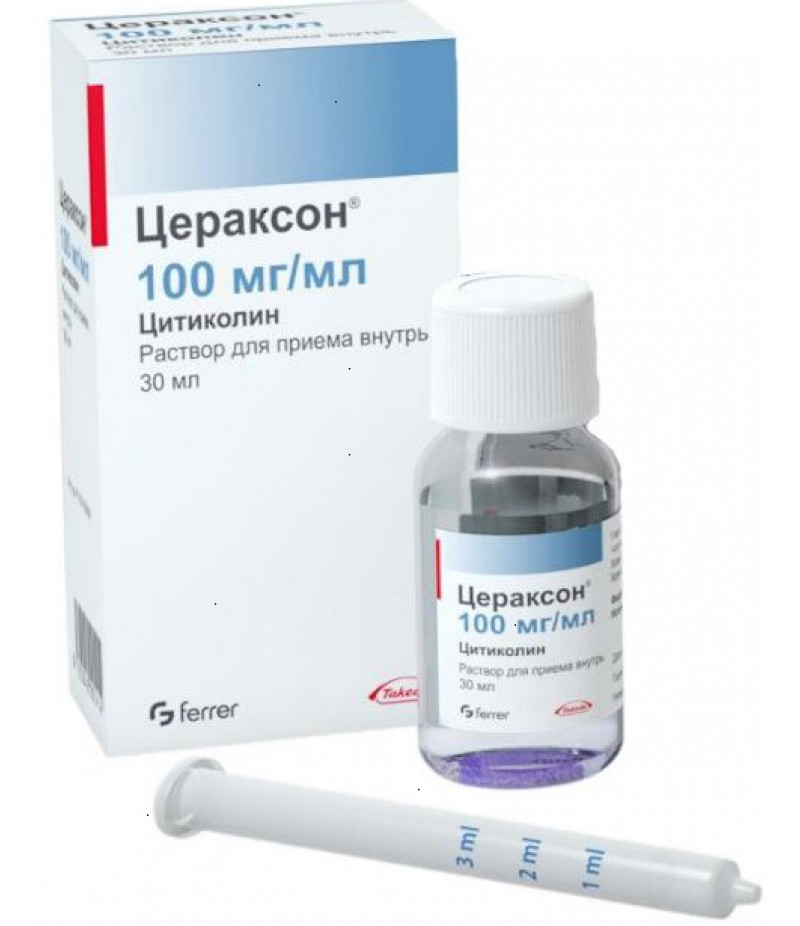
Ceraxon 100mg/ml 30ml
- $37.90
- 2 or more $34.50
- 3 or more $32.90
- Availability:In Stock
Instruction for Ceraxonyou can buy Ceraxon on this pageSolution for parenteral administration Ceraxon represents the clinical and pharmacological group of drugs nootropics. It is used to improve the f
Instruction for Ceraxon
you can buy Ceraxon on this page
Solution for parenteral administration Ceraxon represents the clinical and pharmacological group of drugs nootropics. It is used to improve the functional state of the structures of the central nervous system.
Description of dosage form, composition
Solution for parenteral administration Ceraxon is a colorless transparent liquid. The main active ingredient of the drug is sodium cicoline, its content in 4 ml of the solution is 500 and 1000 mg. Also, the solution contains auxiliary components, which include sodium hydroxide, hydrochloric acid and water for injection. The solution for injections of Ceraxon is contained in glass ampoules of 4 ml, which are packaged in a planar cell pack of 5 packs. The cardboard pack contains one contour mesh package with ampoules and an annotation to the preparation.
Pharmacodynamics, pharmacokinetics
The main active ingredient of the solution for injection Ceraxon cicoline is a key structural component of the cell membranes of the neuronal cells (cells of the nervous system), in particular phospholipids. It promotes their recovery, due to which the functional activity of the central nervous system structures is improved, and several therapeutic effects are realized:
Restoration of damaged membranes of neurocytes.
Suppression of the activity of enzymatic systems responsible for the cleavage of phospholipids (enzymes of phospholipase).
Prevent the formation of an excessive amount of free radicals, which are fragments of organic molecules containing unpaired electrons, which possess high chemical activity and lead to damage to the cell membranes of the neurocytes.
Effects on the mechanism of apoptosis with the prevention of premature death of neurocytes.
Due to such therapeutic effects, the drug reduces the volume of dead brain tissue in an acute period of stroke, reduces the severity of neurological disorders after a craniocerebral trauma, and also reduces the duration of the recovery period. Solution for injection Ceraxon positively affects the cognitive function of the brain in chronic hypoxia (insufficient intake of oxygen into the neurocytes), in particular improves memory, consciousness, concentration, mental performance. The drug has a positive therapeutic effect on motor and sensory disorders, the development of which was provoked by a change in the functional activity of the brain.
After intramuscular or intravenous injection of a solution for injection of Ceraxon, the active ingredient quickly enters the systemic bloodstream, is evenly distributed in the tissues, penetrates the structures of the central nervous system through the blood-brain barrier, where it has a therapeutic effect. It is partially metabolized in the liver with the formation of degradation products that are excreted in the urine. In general, only 15% of the entire administered dose of this drug is excreted from the body, the rest is involved in the metabolism of brain cells.
Indications
The main medical indication for the parenteral administration of the Ceraxon solution is the complex therapy of various pathological conditions affecting the structures of the central nervous system:
Restoration of the functional state of the brain after a previous ischemic or hemorrhagic stroke.
Complex therapy of acute period of ischemic stroke.
Treatment of acute and recovery period after a craniocerebral injury.
The drug is also used for complex therapy of behavioral and cognitive disorders in the elderly, developing against the background of degenerative-dystrophic and vascular pathology of the brain.
Contraindications
Parenteral administration of the solution for injections of Ceraxon is excluded at certain pathological and physiological conditions of the patient's organism, which include:
Expressed vagotonia is a state of significant increase in the functional activity of the parasympathetic part of the autonomic nervous system.
The patient's age is up to 18 years, as there are no reliable data on the safety and efficacy of the drug.
Individual intolerance to any of the components of this medication.
Before prescribing a solution for parenteral administration of Ceraxon, the doctor necessarily excludes the presence of contraindications in the patient.
Dosage
The Ceraxon solution is intended for parenteral intramuscular or intravenous administration. It is injected intramuscularly slowly, for 3-5 minutes. For intravenous administration, the preparation is allowed to dissolve in a physiological solution of sodium chloride (200-250 ml), followed by a droplet injection at a rate of 40-60 drops per minute. More preferred is the intravenous drip administration of this drug. During the manipulation it is very important to follow the rules of aseptic and antiseptic, aimed at preventing infection of the patient. The dosage and mode of use of the Ceraxon injection solution depend on the medical indications:
Acute period of a stroke or craniocerebral trauma - the recommended therapeutic dosage is 1000 mg every 12 hours for a period of at least 6 weeks. After 3-5 days, it is possible to switch to oral dosage forms of the drug.
Recovery period after a stroke, a traumatic brain injury - the recommended therapeutic dosage can vary from 500 to 2000 mg, the drug is administered 1-2 times a day.
If necessary, the doctor determines the mode of administration, dosage and duration of therapy with the solution for injection of Ceraxon individually, depending on the severity of the pathological process.
Side effects
In general, the solution for injecting Ceraxon is well tolerated. It is very rare (1 case per 1000 injections of the drug) to develop allergic reactions (skin rash, itching, hives, angioedema Quincke), insomnia, nausea, vomiting, dizziness, diarrhea, headache, tremor (hand trembling), hallucinations, peripheral edema, dyspnea, decreased appetite. In connection with the effect of the drug on the parasympathetic part of the nervous system, a short-term change in the level of systemic arterial pressure is possible. If there are signs of development of negative side reactions, the possibility of further use of the drug is determined only by the attending physician, which depends on their nature and severity.
Features of use
Before prescribing the solution for parenteral administration of Ceraxon the doctor carefully studies the annotation to it, and also draws attention to several features of its proper use:
It is not recommended simultaneous administration of this medication with drugs that contain meclofenoxate in its composition.
Solution for injection Ceraxon may enhance the therapeutic effects of levodopa.
Clinical evidence of the effect of the drug on the developing fetus is limited today, so the use of the Ceraxon solution for pregnant women is not recommended (it is only possible for life indications).
Against the background of the use of this drug is excluded the employment of a potentially dangerous activity that requires a sufficient speed of psychomotor reactions and concentration of attention.
In the pharmacy network, a solution for injecting Ceraxon is dispensed by prescription. Its independent use is excluded without appropriate medical prescriptions, as this can cause negative consequences for health.
Overdose
Since the active ingredient of the solution for parenteral administration of Ceraxon has very low toxicity, to date, cases of overdose in clinical practice have not been documented.
Shelf life, storage
The shelf life of the solution for injections Ceraxon is 3 years. It should be stored in the original packaging, protected from light and moisture, and also inaccessible to children at an air temperature of not more than + 30 ° C.