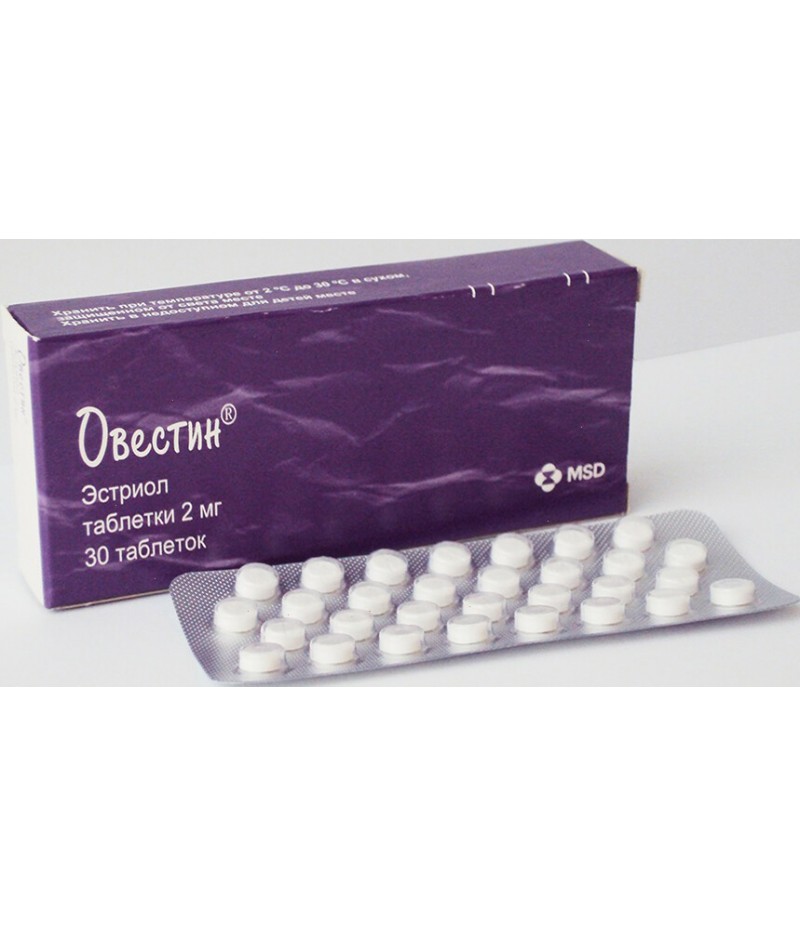
Ovestin tablets 2mg #30
- $49.99$55.90
- Availability:In Stock
Notice: Undefined variable: short_description in /home/innuenze/domains/farmacy-houses.com/public_html/catalog/view/theme/tt_angara3/template/product/product.tpl on line 105
Tags: tabs
Ovestin tablets instruction for use
You can buy Ovestin tablets here
The composition of the drug
Active ingredient: Estriol - 2 mg
Excipients:
Silicon dioxide anhydrous colloid, potato starch, magnesium stearate, povidone, lactose monohydrate, distilled water.
Description:
White round flat tablets with a beveled edge, marked DG above 8, between which there is a separation risk on one side, and ORGANON * on the other side.
Pharmacotherapeutic group:
Estrogen
Pharmacodynamics
Ovestin tablets contains the natural female sex hormone estriol. In the period preceding menopause and postmenopausal (natural or surgical), estriol is used to treat symptoms caused by estrogen deficiency. Estriol has a selective effect mainly on the cervix, vagina, vulva and is particularly effective for the treatment of urogenital symptoms caused by estrogen deficiency. In cases of atrophy of the vaginal mucosa, estriol causes increased proliferation of the epithelium of the vagina and cervix, stimulates its blood supply, helps to restore the epithelium, normal microflora and physiological pH of the vaginal environment, affects the quality and quantity of cervical mucus. As a result, the resistance of epithelial cells to infection and inflammation increases.
Unlike other estrogens, estriol has a short-term effect, since it lingers for a short time in the nuclei of endometrial cells and if the recommended dosing regimen is observed, one should not expect endometrial proliferation. In this regard, the cyclic use of progestogens is not necessary, postmenopausal withdrawal bleeding does not occur.
Pharmacokinetics
After oral administration, estriol is rapidly and almost completely absorbed in the gastrointestinal tract. The maximum concentration of unconjugated estriol in plasma is achieved within 1 hour after administration. About 90% of estriol binds to plasma albumin and, unlike other estrogens, estriol has almost no binding to sex hormone-binding globulin (HSPH). Estriol metabolism consists mainly of conjugation and deconjugation during the enterohepatic circulation. Estriol, the end product of metabolism, is mainly excreted in the urine in conjugated form. Only a small portion (± 2%) is excreted in the feces, mainly in the form of unconjugated estriol.
Indications for use
Atrophy of the lower end of the urogenital tract due to estrogen deficiency, in particular, to treat symptoms such as dyspareunia, vaginal dryness and itching, to prevent recurrent infections of the vagina and lower urinary tract; for the treatment of urinary disorders (eg, increased frequency, dysuria) and moderate urinary incontinence;
Pre- and postoperative treatment during vaginal surgery in the postmenopausal period;
Climacteric disorders, such as hot flashes and night sweats;
As an auxiliary diagnostic tool when receiving an atrophic picture of a cervical smear;
Infertility due to cervical factors.
Contraindications for Ovestin tablets
Pregnancy, lactation;
Hypersensitivity to the active and (or) excipients of Ovestin tablets;
Identified or suspected estrogen-dependent tumors (breast cancer, endometrial cancer);
Vaginal bleeding of unknown etiology;
Confirmed venous thromboembolism (deep vein thrombosis, pulmonary thromboembolism) in the past two years;
Venous thromboembolism in history or thrombosis if anticoagulant therapy is not performed;
Diabetes mellitus with angiopathy;
Sickle cell anemia;
Dubin-Johnson Syndrome;
Violation of cerebral circulation;
Rotor syndrome. Carefully
Familial hyperlipoproteinemia;
Increased risk of thromboembolic complications;
Systemic lupus erythematosus;
Prolonged immobilization, serious surgical interventions;
Severe liver disease;
A history of gallbladder disease (especially cholelithiasis);
Hepatic porphyria;
Severe itching or cholestatic jaundice (including a history of a previous pregnancy);
Pancreatitis;
Endometriosis;
Leiomyoma;
Bronchial asthma;
Arterial hypertension;
Hypercalcemia due to bone metastases of breast cancer;
Herpes pregnant;
Epilepsy;
Otosclerosis.
Dosage and administration
Ovestin tablets are used inside. The daily dose for oral administration should not exceed 8 mg. With atrophy of the lower parts of the urogenital tract due to estrogen deficiency: 4-8 mg per day for the first 4 weeks, followed by a gradual decrease in dose in accordance with the symptoms until a maintenance dose of 1-2 mg per day is reached.
Pre- and postoperative treatment for vaginal operations in the postmenopausal period: 4-8 mg per day for 2 weeks before surgery, 1-2 mg per day for 2 weeks after surgery.
Treatment of menopausal disorders (hot flashes, night sweats): 4–8 mg per week with a gradual decrease in dose. For maintenance therapy, the minimum effective dose should be used.
For infertility due to cervical factors: as a rule, 1-2 mg is administered per day from day 6 to day 15 of the menstrual cycle. However, in different patients, the daily dose may vary from 1 to 8 mg. The dose should be increased each month to achieve an optimal effect on the mucous membrane of the cervix. If a woman missed the next dose, and the delay was no more than 12 hours, you need to take it as soon as possible. If the delay is more than 12 hours, you should skip one dose and then take Ovestin tablets at regular times.
Tablets are taken with water, preferably at the same time of day. The daily dose should be taken in one step.
Side effect of Ovestin tablets
Soreness and tension of the mammary glands, jaundice, nausea, skin rash, increased blood pressure, headache, intermenstrual bloody spotting from the vagina, cervical hypersecretion. Adverse reactions are usually transient in nature, but may also indicate an overdose of Ovestin tablets.
Overdose
Overdose causes nausea, vomiting and vaginal bleeding.
Symptomatic treatment.
Interaction with other drugs
There were no cases of drug interactions with Ovestin tablets other drugs. At the same time, there are data on the enhancement of the pharmacological effect of glucocorticosteroids, lipid-lowering agents when used together with estrogens. If necessary, the dose of glucocorticosteroids can be reduced.
Possible weakening of the effects of drugs of male sex hormones, anticoagulants, antidepressants, diuretic, hypotensive and hypoglycemic drugs.
Barbiturates, antiepileptic drugs (carbamazepine, phenytoin) increase the metabolism of steroid hormones.
Antibiotics (ampicillin, rifampicin), drugs for general anesthesia, narcotic analgesics, anxiolytics, antiepileptic drugs, some antihypertensive drugs, ethanol reduce the effectiveness of estrogens.
Folic acid and thyroid hormone drugs enhance the effect of estriol.
Estriol can change the effectiveness of oral anticoagulants, increase the pharmacological effect of succiniccholine, theophylline, foleandomycin.
special instructions
Before starting hormone replacement therapy, a full medical examination is necessary. During treatment, every 6 months, regular examinations should be carried out (including breast examinations, mammography) in accordance with accepted medical practice. It is necessary to exclude the presence in the history of thromboembolism, repeated spontaneous abortions, which indicates thrombophilia. The risk of thromboembolism increases with prolonged immobilization, severe injuries and surgical interventions. In these cases, it is necessary to temporarily interrupt hormone replacement therapy (4-6 weeks before surgery)
The use of estriol does not increase the density of the breast. And perhaps the use of estriol does not increase the risk of developing breast cancer.
Cases of venous thromboembolism (deep vein thrombosis of the lower leg, pelvic vein thrombosis, and pulmonary thromboembolism) are more common in women who receive hormone replacement therapy. In relation to the drug Ovestin such data are not available, so it is not known whether its use causes an increase in the incidence of venous thromboembolism. However, it is recommended that you follow the guidelines outlined in the "Contraindications" section.
Release form
Tablets 2 mg. On 30 tablets in the blister from PVC / A1. On 1 blister with the application instruction place in a cardboard box.
Storage conditions
At a temperature of 2-30 ° C, protected from light and moisture and inaccessible to children. Shelf life - 5 years.
Do not use after the period indicated on the package.
Pharmacy sales terms
You don't need a prescription to buy Ovestin tablets.