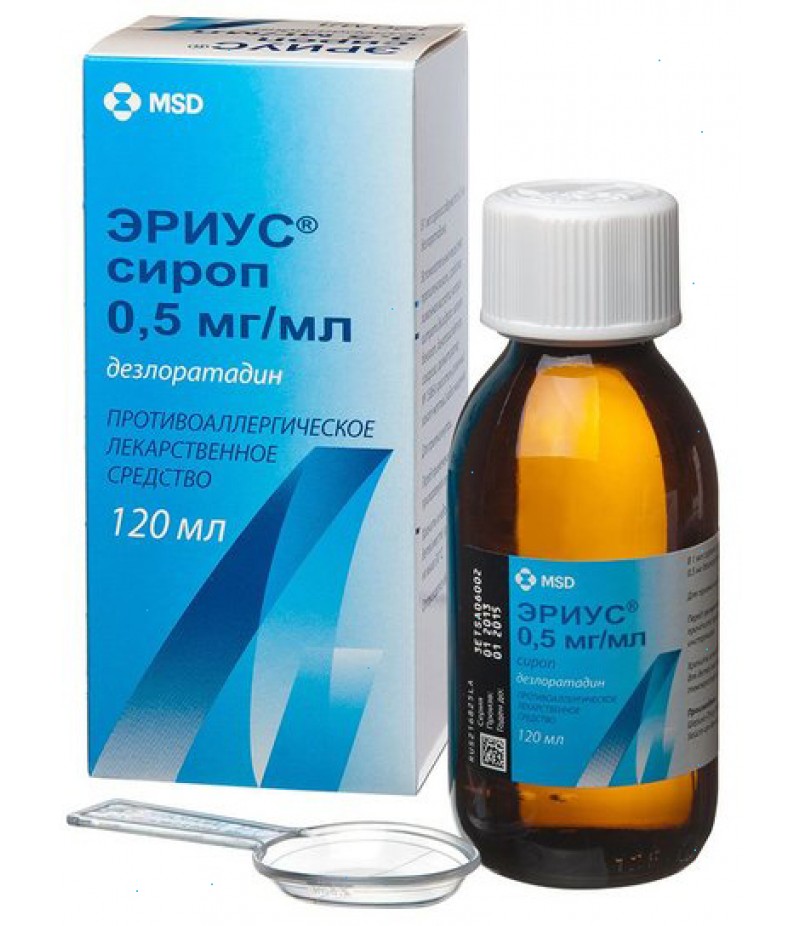
Aerius syrup 0.05% 60ml
- $35.87
- Availability:In Stock
Aerius syrup instuction for useYou can buy Aerius syrup hereClinical and pharmacological groupThe blocker of histamine H1 receptors. Anti-allergic drugForm of release, composition and packagingOrange syrup, transparent.1 ml deslor..
Aerius syrup instuction for use
You can buy Aerius syrup here
Clinical and pharmacological group
The blocker of histamine H1 receptors. Anti-allergic drug
Form of release, composition and packaging
Orange syrup, transparent.
1 ml desloratadine (micronized) 500 μg
Auxiliary
substances: propylene glycol 100 mg, sorbitol 150 mg, citric acid 500
μg, sodium citrate dihydrate 1.26 mg, sodium benzoate 1 mg, disodium
edetate 250 μg, sucrose 490 mg, flavor No. 15864 750 μg, dye sunset sunset yellow - 23 mcg, purified water - up to 1 ml.
60 ml - bottles of dark glass (1) complete with a dosage spoon, graduated to 2.5 ml - packs of cardboard.
pharmachologic effect
Antihistamine is a long-acting drug, a blocker of peripheral histamine H1 receptors. Desloratadine is the primary active metabolite of loratadine. Inhibits a cascade of reactions of allergic inflammation, incl. release
of pro-inflammatory cytokines, including interleukins IL-4, IL-6, IL-8,
IL-13, release of pro-inflammatory chemokines, production of superoxide
anions by activated polymorphonuclear neutrophils, adhesion and
chemotaxis of eosinophils, isolation of adhesion molecules such as
P-selectin, IgE- mediated release of histamine, prostaglandin D2 and leukotriene C4. Thus, it prevents development and facilitates the course of allergic
reactions, has antipruritic and antiexudative action, reduces
permeability of capillaries, prevents the development of edema of
tissues, spasm of smooth muscles.
The drug has no effect on the central nervous system, has almost no
sedative effect (does not cause drowsiness) and does not affect the rate
of psychomotor reactions.
Does not cause an extension of the QT interval on the ECG.
The action of the preparation of Eryus begins within 30 minutes after ingestion and lasts for 24 hours.
Pharmacokinetics
Suction
After taking the drug inside desloratadine is well absorbed from the
gastrointestinal tract, with determined concentrations of desloratadine
in the blood plasma reached within 30 minutes, and Cmax - after about 3
hours.
Distribution
The binding of desloratadine to plasma proteins is 83-87%. When
used in adults and adolescents for 14 days at a dose of 5 mg to 20 mg 1
time / day, no signs of a clinically significant cumulation of
desloratadine have been detected. Simultaneous
food intake or simultaneous consumption of grapefruit juice does not
affect the distribution of desloratadine (when taken at a dose of 7.5 mg
1 time / day). Does not penetrate the BBB.
Metabolism
Exposed to intensive metabolism by hydroxylation to form 3-OH-desloratadine, coupled with glucuronide. It is not an inhibitor of CYP3A4 and CYP2D6 and is not a substrate or an inhibitor of P-glycoprotein.
Excretion
Desloratadine is excreted from the body in the form of a glucuronide
compound, and only a small part is excreted by the kidneys (<2%) and
through the intestine (<7%) unchanged.
T1 / 2 averages 27 hours.
Indications
- allergic rhinitis (elimination or relief of sneezing, nasal
congestion, discharge of mucus from the nose, itching of the nose,
itching of the palate, itching and redness of the eyes, lachrymation);
- urticaria (reduction or elimination of itching, rash).
Contraindications
- Pregnancy;
- lactation (breastfeeding);
- age to 1 year (for syrup);
- age up to 12 years (for coated tablets);
- hereditary transmitted diseases (fructose intolerance, impaired
glucose / galactose absorption or sugarase / isomaltase insufficiency in
the body) due to the presence of sucrose and sorbitol in the syrup;
- Hypersensitivity to the components of the drug.
With caution should be used in patients with severe renal failure.
Dosage
Syrup
Children aged 1 to 5 years are prescribed 1.25 mg (2.5 ml syrup) 1 time / day; children aged 6 to 11 years - 2.5 mg (5 ml syrup) 1 time / day; adults and teenagers over 12 years - 5 mg (10 ml of syrup) 1 time / day.
Syrup is taken orally, regardless of food intake, with a small amount of water.
Side effects
Syrup
In
children under 2 years of age, the following adverse events were
observed with the use of the Erius preparation, the frequency of which
was slightly higher than in placebo (≥1 / 100 to <1/10): diarrhea
(3.7%), body temperature increase (2.3% ), insomnia (2.3%).
In children aged 2 to 11 years with the use of Erius, the incidence of side effects was the same as with placebo.
In
adults and adolescents aged 12 years and older with the use of the
Erius preparation, the incidence of side effects was slightly higher
compared with placebo (≥1 / 100 to <1/10), the most frequent of which
were fatigue, dry mouth, headache .
When using Aerius in adults and adolescents at a recommended dose of 5
mg / day, the incidence of drowsiness was not higher than with placebo.
Very rarely (<1/10 000), the following side effects were noted.
From the side of the central nervous system: dizziness, drowsiness.
From the cardiovascular system: tachycardia, palpitations.
On the part of the digestive system: abdominal pain, nausea, vomiting,
dyspepsia, diarrhea, increased bilirubin, hepatic enzymes in the blood
serum.
Allergic reactions: anaphylaxis, angioedema, itching, rash, urticaria.
Overdose
Symptoms: taking a dose that exceeds the recommended dose 5 times did not lead to any symptoms.
In
clinical trials, the daily use of desloratadine in adults and
adolescents at doses up to 20 mg for 14 days was not accompanied by
statistically or clinically significant changes in the cardiovascular
system. In a clinical pharmacological study, the use of desloratadine at a
dose of 45 mg / day (9 times higher than recommended) for 10 days did
not cause prolongation of the QT interval and was not accompanied by
serious side effects.
Treatment: with the occasional ingestion of a large amount of the drug - gastric lavage, the reception of activated charcoal; if necessary, symptomatic therapy. Desloratadine is not excreted in hemodialysis, the effectiveness of peritoneal dialysis is not established.
Drug Interactions
In studies, no interaction with azithromycin, ketoconazole, erythromycin, fluoxetine, and cimetidine has been identified.
Aerius does not increase the effects of ethanol on the central nervous system.
Eating does not affect the effectiveness of the drug.
special instructions
There were no studies of the efficacy of Erius in rhinitis of infectious etiology.
Use in Pediatrics
The effectiveness and safety of Erius in the form of syrup in children under the age of 1 year is not established.
Influence on the ability to drive and drive machinery
In the recommended dose, Erius does not affect the ability to drive vehicles or control mechanisms.
Pregnancy and lactemia
The use of the drug in pregnancy is not recommended due to the lack of clinical data on the safety of its use in this period.
Desloratadine is excreted in breast milk, so the use of Erius during breastfeeding is not recommended.
Application in childhood
The effectiveness and safety of Erius application in the form of syrup in children under the age of 1 year is not established.
Contraindicated at the age of up to 1 year (for syrup), at the age of 12 years (for coated tablets).
In most cases, rhinitis in children under 2 years of age is of an infectious nature. There were no studies of the efficacy of Erius in rhinitis of infectious etiology.
Differential
diagnosis between allergic rhinitis and rhinitis of a different origin
in children under 2 years of age presents certain difficulties. When
making a differential diagnosis, attention should be paid to the
presence or absence of foci of infection or structural anomalies of the
upper respiratory tract, to conduct a careful history, examination, as
well as appropriate laboratory tests and skin tests.
In case of violations of kidney function
With caution should be used in patients with severe renal failure.
Conditions of leave from pharmacies
The drug is approved for use as a means of OTC.
Terms and conditions of storage
The drug should be stored out of reach of children at a temperature of no higher than 30 ° C. Shelf life - 2 years.