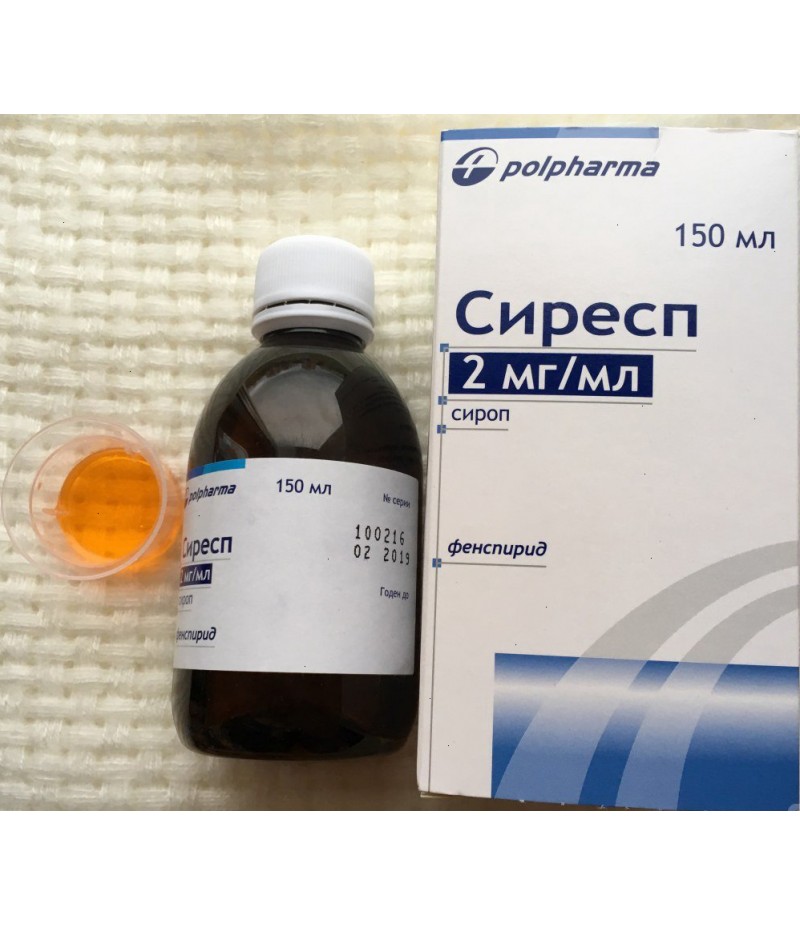
Eurespal (Erespal) syrup 2mg/ml 150ml
- $13.35
- 3 or more $11.90
- Availability:Out Of Stock
Eurespal (Erespal) syrup instructionYou can buy Eurespal syrup hereClinical and pharmacological groupThe drug with anti-inflammatory and anti-bronchoconstrictive activityForm of release, composition and packagingSyrup in the form ..
Eurespal (Erespal) syrup instruction
You can buy Eurespal syrup here
Clinical and pharmacological group
The drug with anti-inflammatory and anti-bronchoconstrictive activity
Form of release, composition and packaging
Syrup in the form of a liquid of a brownish-yellow color, transparent or with slight opalescence; during storage, the formation of a precipitate that disappears with agitation is possible.
100 ml
fenspiride hydrochloride 200 mg
Auxiliary substances: flavoring composition with hints of honey smell (flavors, including natural, sunflower, water, ethanol *) - 500 mg, licorice root extract - 200 mg, vanilla tincture (natural vanilla flavor and other components, t ethanol *) 400 mg, glycerol 22.5 g, methyl parahydroxybenzoate 90 mg, propyl parahydroxybenzoate 35 mg, saccharin 45 mg, sucrose 60 g, potassium sorbate 190 mg, purified water 100 ml.
* - the total content of ethanol in 100 ml of syrup - no more than 0.29 mg.
150 ml - plastic bottles of brown color (1) - packs cardboard.
pharmachologic effect
Anti-inflammatory drug, has an antiexudative effect, interferes with the development of bronchospasm. It shows antagonism with mediators of inflammation and allergy: serotonin, histamine (blockade of H1-histamine receptors), bradykinin. Has spasmolytic miotropic effect.
When administered in large doses reduces the production of various inflammation factors (cytokines, arachidonic acid derivatives, free radicals).
Pharmacokinetics
Suction
Well absorbed from the digestive tract. After oral administration, Cmax is achieved in 2.3 ± 2.5 h (0.5 to 8 h).
Excretion
T1 / 2 is 12 hours. It is excreted mainly by the kidneys (90%), through the intestine 10%.
Indications
Diseases of the upper and lower respiratory tract:
- Rhinopharyngitis and laryngitis;
- tracheobronchitis;
- bronchitis (in the background of chronic respiratory failure or without it);
- bronchial asthma (as part of complex therapy);
- respiratory effects (cough, hoarseness, swelling in the throat) in measles, whooping cough, influenza;
- infectious diseases of the respiratory tract accompanied by cough, when standard antibiotic therapy is indicated.
Otitis and sinusitis of various etiologies.
Contraindications
- Children under 2 years;
- Hypersensitivity to the active substance and / or any of the components of the drug.
With caution: patients with fructose intolerance, glucose-galactose malabsorption, sugarase / isomaltase deficiency (due to the presence of sucrose in the Eurespal syrup), patients with diabetes mellitus (due to the presence of sucrose as part of Eurespal syrup).
Dosage
Follow strictly the recommendations of the attending physician.
Inside.
For adults and adolescents, the drug is prescribed 3-6 table spoons (45-90 ml) / day, taken before meals.
For children from 2 years of age, the drug is prescribed at the rate of 4 mg / kg body weight / day. Children with a body weight of up to 10 kg - 2-4 teaspoons (10-20 ml) / day, can be added to a bottle with food. Children with a body weight of more than 10 kg - 2-4 tablespoons (30-60 ml) / day, take before meals.
Syrup before use should be shaken.
1 tablespoon (15 ml of syrup) contains 30 mg of fenspiride hydrochloride and 9 g of sucrose.
1 teaspoon (5 ml of syrup) contains 10 mg of fenspiride hydrochloride and 3 g of sucrose.
The duration of treatment is determined by the doctor.
Side effects
The most frequent adverse reactions to Eurespal are observed on the part of the digestive system.
The frequency of adverse reactions that may occur during therapy is given in the following gradation: very often (> 1/10); often (> 1/100, <1/10); infrequently (> 1/1000, <1/100); rarely (> 1/10 000, <1/1000); very rarely (<1/10 000); an unknown frequency (the frequency can not be calculated from the available data).
From the cardiovascular system: rarely - a moderate tachycardia, the severity of which decreases with a decrease in the dose of the drug; unknown frequency * - palpitation, hypotension, possibly associated with tachycardia.
On the part of the digestive system: often - gastrointestinal disorders, nausea, epigastric pain; unknown frequency * - diarrhea, vomiting.
From the side of the central nervous system: rarely - drowsiness; unknown frequency * - dizziness.
From the skin and subcutaneous fat: rarely - erythema, rash, hives, angioedema, fixed erythema pigmentosa; unknown frequency * - skin itch, toxic epidermal necrolysis, Stevens-Johnson syndrome.
General disorders: an unknown frequency * - asthenia, increased fatigue.
* Data of post-registration application.
The patient should be informed of the need to inform the doctor about any, including. not mentioned above undesirable reactions, as well as the change in laboratory indicators against the background of Eurespal therapy.
Overdose
When an overdose of the drug (noted cases of overdose when taking the drug at a dose of more than 2320 mg), the patient should immediately seek medical help.
Symptoms: drowsiness or agitation, nausea, vomiting, sinus tachycardia.
Treatment: gastric lavage, ECG monitoring, maintenance of vital body functions.
Drug Interactions
Special studies on the interaction of fenspiride with other drugs have not been conducted.
Because of the possible sedative effect when taking blockers of histamine H1 receptors, the use of Eurespal syrup in combination with drugs with sedative effect or with alcohol is not recommended.
special instructions
The composition of the preparation in the form of a syrup includes parabens (parahydroxybenzoates), as a result of which this drug can provoke the development of allergic reactions, including. deferred.
When prescribing the drug to patients with diabetes it is necessary to take into account that Eurespal syrup contains sucrose (1 teaspoon - 3 g sucrose = 0.3 XE, 1 tablespoon - 9 g sucrose = 0.9 XE).
Impact on the ability to drive vehicles and manage mechanisms
Studies to study the effect of Eurespal on the ability to drive vehicles and work with mechanisms have not been carried out. Patients should be aware of the possible development of drowsiness when taking Eurespal, especially at the beginning of therapy or when combined with alcohol intake, and should exercise caution when driving vehicles and performing work that requires a high rate of psychomotor reactions.
Pregnancy and lactemia
Data on the use of Eurespal in pregnant women are absent or limited. The use of the drug in pregnancy is not recommended.
Therapy with fenspiride is not a reason for interrupting the pregnancy that has occurred.
Do not use Eurespal during breastfeeding due to the lack of data on the penetration of fenspiride into breast milk.
Application in childhood
Contraindicated in children under 2 years.
Terms and conditions of storage
The drug should be stored out of the reach of children. Special storage conditions are not required. Shelf life - 3 years. Do not use after the expiration date printed on the package.